In collaboration with the Manke Lab at UMass Dartmouth and the Designer Drug Research Unit (DDRU) at the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP), CaaMTech scientists continue to pioneer new fields of “magic mushroom” research with their latest published academic work, “Synthesis, Structural Characterization, and Pharmacological Activity of Novel Quaternary Salts of 4-Substituted Tryptamines.” Published in the American Chemical Society’s journal, ACS Omega (Structural Chemistry), the multi-disciplinary peer-reviewed research paper builds on the researchers’ previous synthesis, structural characterization, and pharmacological screening of aeruginascin’s active metabolite (4-HO-TMT) and a prospective prodrug thereof (4-AcO-TMT).
Psilocybin is the most studied compound in “magic mushrooms.” now the subject of over 100 clinical trials. But a growing body of evidence indicates that psilocybin is not the only biologically important compound in magic mushrooms. Recent studies have highlighted the prevalence of other, structurally similar tryptamine compounds in magic mushrooms, including baeocystin, norbaeocystin, norpsilocin, aeruginascin, and 4-HO-TMT. Improving our understanding of these lesser-known tryptamines is essential to understanding the pharmacology of magic mushrooms and how it compares to pure psilocybin.
CaaMTech’s latest research broadens the scientific understanding of aeruginascin and 4-HO-TMT by comparing a series of structurally similar compounds, i.e. quaternary tryptammonium compounds, aka “quats,” in an effort to develop a structure activity relationship. The current publication illustrates how small modifications in the chemical structure of these compounds correlate with significant changes in pharmacology.
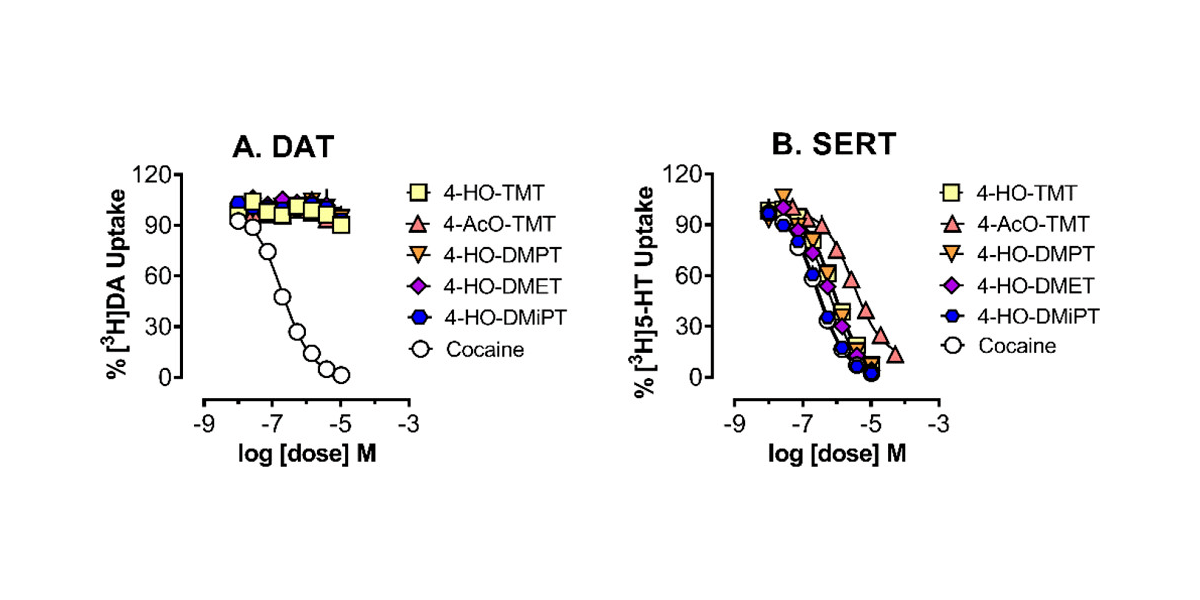
In the paper, CaaMTech scientists report the synthesis and crystallographic characterization of three quaternary tryptammonium compounds similar in structure to naturally occurring 4-HO-TMT: 4-HO-DMET, 4-HO-DMPT, and 4-HO-DMiPT. The researchers further reported the design, synthesis, and chemical characterization of novel prodrugs for each: 4-AcO-DMET, 4-AcO-DMPT, and 4-AcO-DMiPT, respectively. The researchers then screened the compounds for in vitro pharmacological activity at 46 receptors and transporters. For each compound having substantial binding affinity at a particular receptor, further functional assays were conducted for purposes of measuring agonist potency at 5-HT1D and 5-HT2B, as well as uptake inhibition potency at DAT and SERT receptors.
The authors of the paper concluded that for the compounds studied, “the most substantial binding affinity and noteworthy SAR was observed at SERT.” The data for these compounds further showed that SERT binding affinity and potency for uptake inhibition were both increased by steric bulking of the quaternary ammonium unit. Competitive radioligand binding assays also revealed in vitro binding affinity for some of the compounds at 5-HT1D, 5-HT2B, and DAT.
“Two years ago, no one appreciated that aeruginascin was present in magic mushrooms,” explained CaaMTech CEO, Dr. Andrew Chadeayne. “Since then, we’ve found it in the most widespread species [Psilocybe cubensis], highlighting the importance of its pharmacology–and how it might modulate the pharmacology of psilocybin.”